Nice Tips About How To Tell If A Chemical Reaction Is Endothermic Or Exothermic
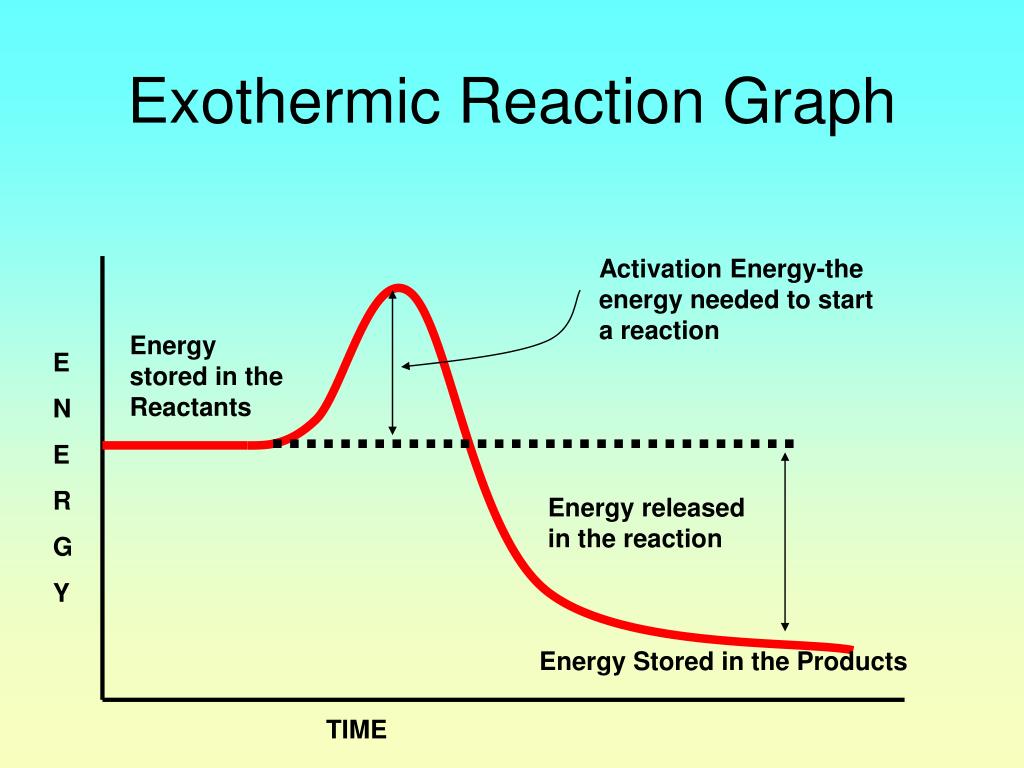
On the other hand, an exothermic reaction.
How to tell if a chemical reaction is endothermic or exothermic. The changes in energy that occur during a chemical reaction can be seen. Decomposition reactions can be exothermic or endothermic, depending on the chemical energy of the substances. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to.
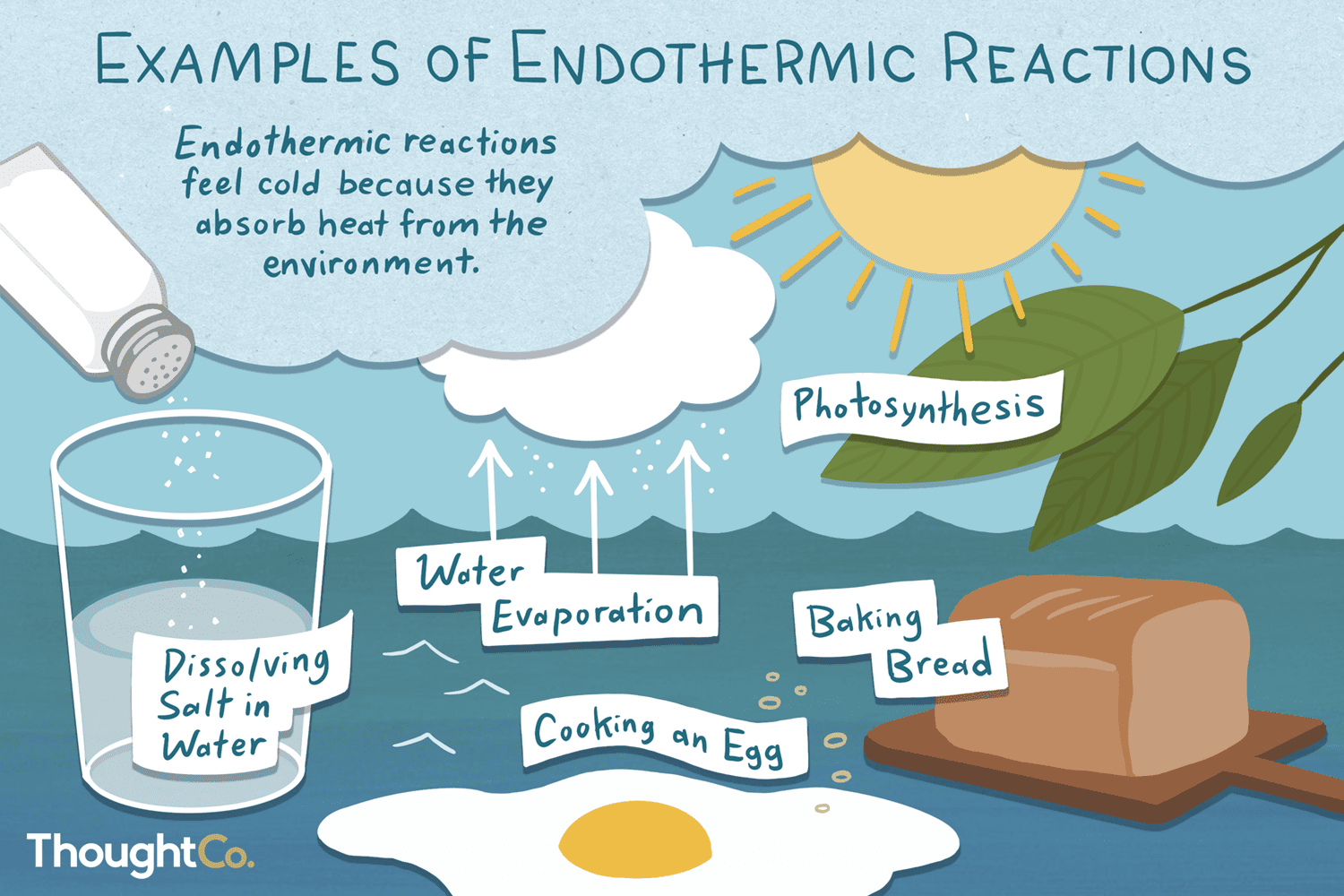
Photosynthesis is an endothermic reaction because sunlight is being absorbed during the reaction and the definition suggests that in a reaction if heat is being absorbed it is. Is this an exothermic or endothermic reaction? The heat is absorbed from the surroundings.
What is an endothermic reaction? In simple terms, the endothermic reactions absorb energy from the surrounding that is in the form of heat. We can use the law of conservation of energy to determine.
Are these reactions endothermic or exothermic? If the chemical energy of reactants are greater than products. In endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or.
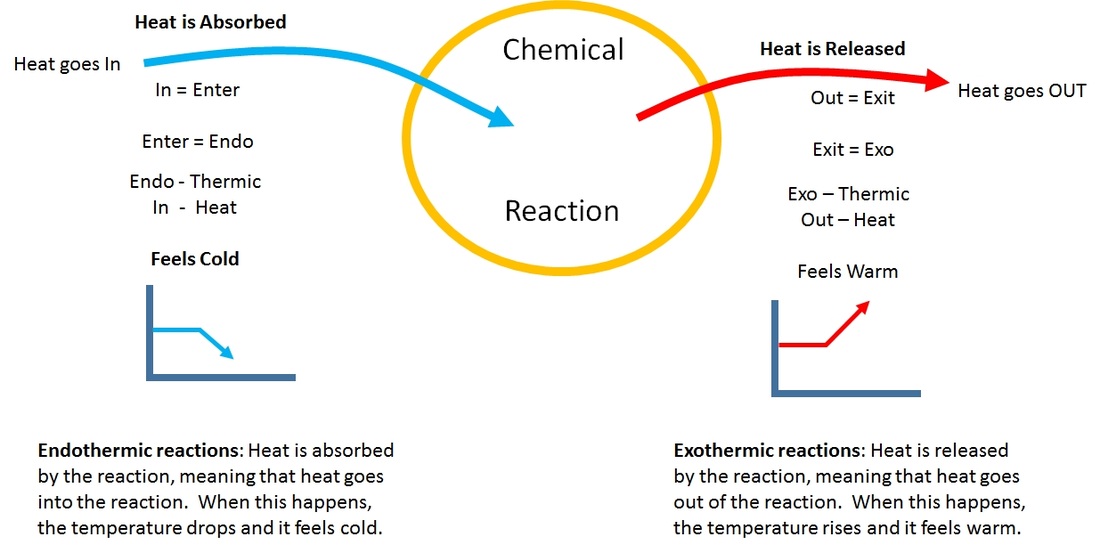
Endothermic reactions require energy, so energy is a reactant. When a chemical reaction happens, energy is transferred to or from the surroundings. Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product.
Actually, i want to know if there is some way we can decide whether a reaction is endothermic or exothermic just by looking at the equation? When energy is transferred to the surroundings, this is called an. Instant cold packs use chemical reaction.
For only £3.50 you will receive all five revision mats, which include: Describe three ways to speed up a reaction. How do you tell if a reaction is exothermic or endothermic?
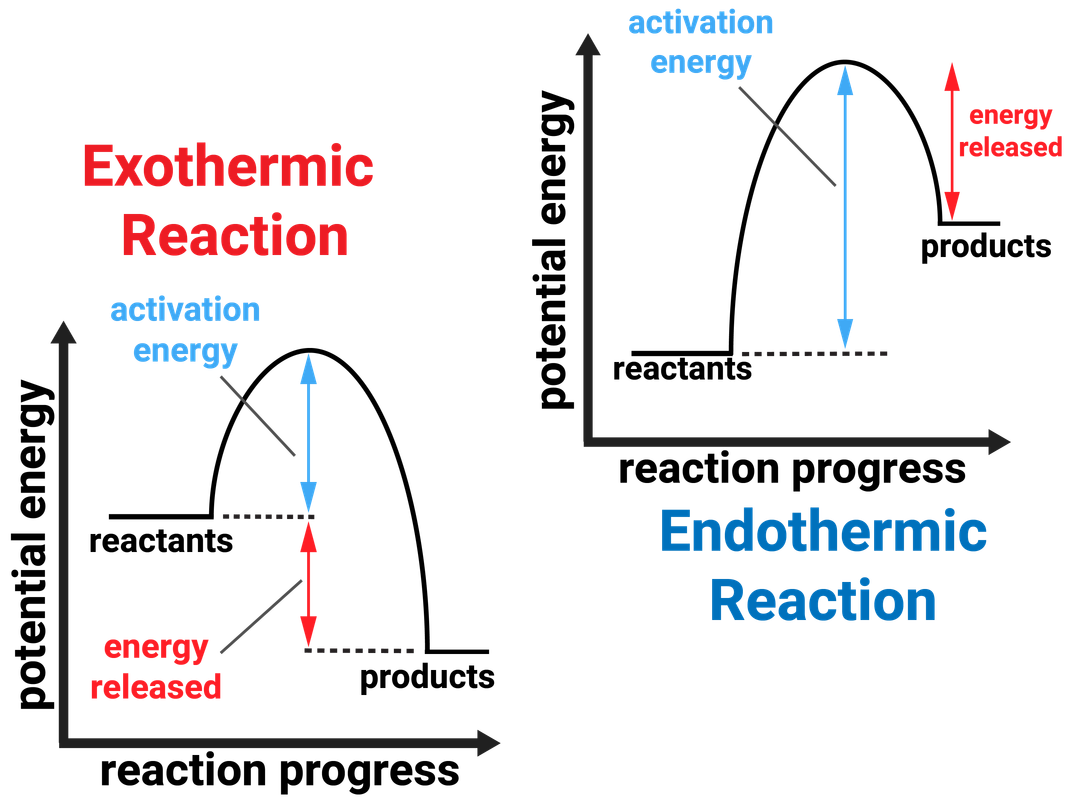
* bond energy calculations * electrolysis * electrolysis of aluminium oxide * endothermic and. Chemical reactions can result in energy being released (exothermic) or energy being absorbed (endothermic). In a chemical reaction, if the sum of the enthalpies of all the reactants is greater than the sum of the.
If δh is negative, the process releases heat to the surroundings and is. Identifying endothermic and exothermic reaction. In the following reaction, the temperature is increased and the \(k_ c\) value decreases from 0.75 to 0.55.
Heat flows from the surroundings to the system (reaction mixture) and the. In chemistry, endothermic and exothermic only consider the change in enthalpy (a measure of the total energy of the system); A chemical reaction is said to be endothermic when it absorbs energy, mostly heat.
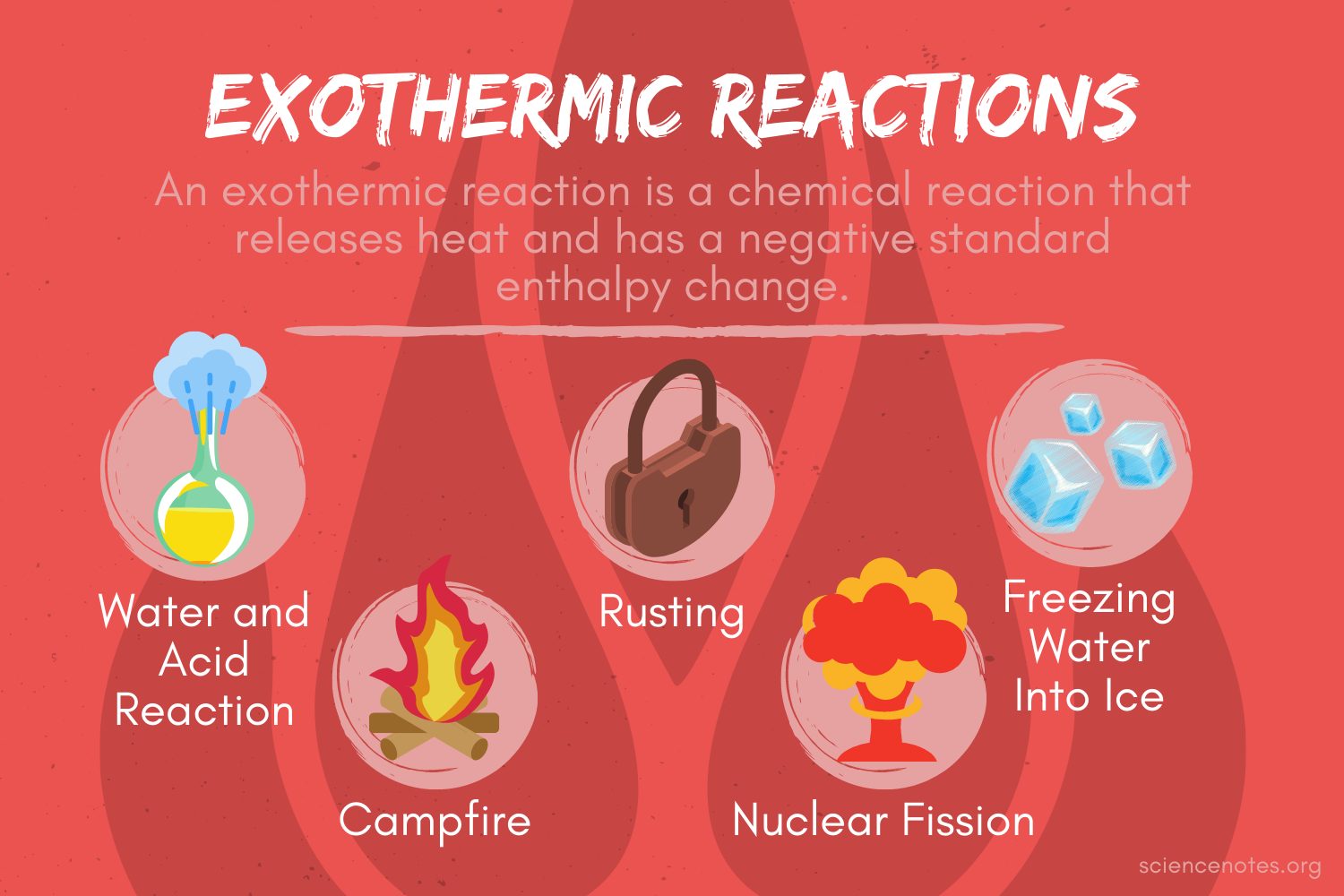
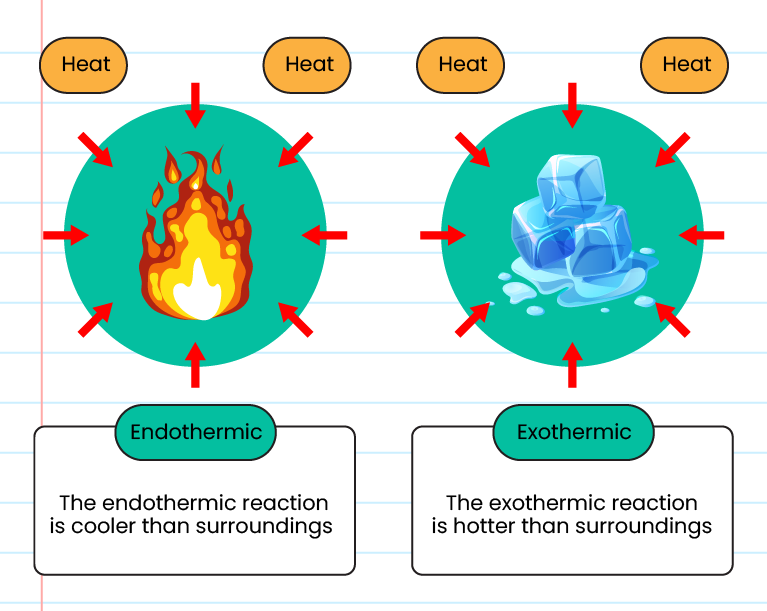








:max_bytes(150000):strip_icc()/endothermic-and-exothermic-reactions-602105_final-c4fdc462eb654ed09b542da86fd447e2.png)
:max_bytes(150000):strip_icc()/types-of-chemical-reactions-604038_FINAL-728e463b035e4cca84544ed459853d5c.png)